News release, UBC and VCH | January 16, 2018
The University of British Columbia and Vancouver Coastal Health are testing a possible diabetes cure that replaces a person’s damaged pancreatic cells with new ones grown in the lab.
The replacement cells are grown from embryonic stem cells. Researchers believe that if the new cells mature, multiply, and behave as hoped, recipients would be able to lessen – or even eliminate – their dependence on self-injected insulin. They might also be spared from having to continually monitor their blood sugar, usually by pricking their fingers several times a day.
“If these replacement cells restore a person’s ability to produce their own insulin when needed, it would prevent dangerous episodes of low blood sugar and lessen the complications resulting from high blood sugar, such as blindness, heart attacks and kidney failure,” said Dr. David Thompson, a principal investigator in the clinical trial, a UBC clinical assistant professor of endocrinology and medical director of the Vancouver General Hospital Diabetes Centre.
“Eventually, it might even free people from a lifetime of constantly checking their blood sugar and injecting themselves, transforming treatment of this disease into a more manageable condition.”
The trial could involve about 10 or more people in Vancouver with a severe form of type 1 diabetes, in which a person’s immune system attacks the pancreas, degrading or eliminating its ability to produce insulin.
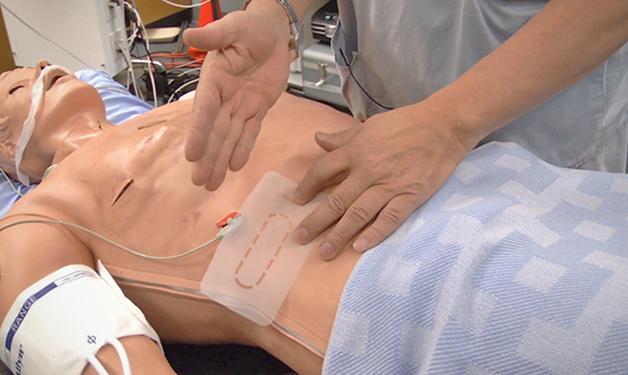
A UBC-Vancouver Coastal Health team, supported by a $500,000 grant from the Stem Cell Network of Canada, implanted the cells into one person in late December at Vancouver General Hospital, and they hope to do more implants in coming weeks. Participants will be followed for two years to see if the implanted cells mature into insulin-producing beta cells and other cells capable of controlling a person’s blood sugar, and whether there are significant side effects.
The implants are part of a larger clinical trial by San Diego-based ViaCyte, a biotechnology company that plans to test the cell-replacement therapy on approximately 40 patients in the U.S. and Canada.
The implanted cells originally came from a single embryonic cell line, a type of cell capable of becoming any other type of cell in the body. ViaCyte has developed a technique for coaxing stem cells along a path to becoming immature pancreatic cells.
Tim Kieffer, a UBC professor of cellular and physiological sciences, demonstrated in 2012 that implanting stem cell-derived pancreatic cells like these in diabetic mice reversed the disease, effectively re-creating the “feedback loop” that enables insulin levels to automatically rise or fall based on blood-glucose levels.
For the human clinical trial, ViaCyte also has developed a protective packet that is implanted just below the skin. The packet’s membrane allows blood vessels to penetrate inside, so the millions of cells within can be bathed in oxygen and other nutrients, spurring them to differentiate further. The researchers expect some of the cells will become beta cells, which sense blood sugar levels and release insulin when needed.
Several packets of cells, each about half the size of a business card, are implanted for up to two years. Smaller “sentinel” packets, about the size of a dime, are also implanted, and will be removed at earlier points so physicians and scientists can examine the packets’ condition and development of the cells inside.
To decrease chances that participants’ bodies will reject the units, they will take drugs to suppress their immune systems. However, this will also make them more vulnerable to infections. Because of the risks, the trial is being limited to people who have a particularly dangerous form of type 1 diabetes.
The procedure for implanting the cells, performed by a team led by Dr. Garth Warnock, a UBC surgery professor, is similar to transplanting clusters of beta cells, known as “islets,” from deceased donors, a treatment pioneered at the University of Alberta and that is still being refined. But donor cells are scarce – only 1,000 people have received such transplants have been done worldwide – while stem cells can be continually grown in the lab. That means there is a virtually endless supply of cells to treat type 1 diabetes, a disease that affects about 300,000 Canadians and 1.25 million Americans.